partner
OriMIRACLE S™ Minimal Residual Disease (MRD) Dynamic Monitoring
-

WES + MRD Testing
Whole Exome Sequencing (WES) and Minimal Residual Disease (MRD) are techniques allowing matching of the genetic sequences detected in your tumor biopsy to 20,000 known genes from our database
-

Tumor Specific MRD Monitoring
Minimal Residual Disease refers to the small number of residual cancer cells circulating in the blood after cancer treatment. Detection of any remaining circulating cancer cells means there is MRD, or ongoing untreated cancer. Our MRD testing accurately detects any remaining cancer cells, indicating successful treatment if all cancer cells disappear from the circulation
-

Ongoing Monitoring Against Recurrence
The same technology used for MRD can also be used to detect tumor recurrence and for ongoing monitoring of patients who have been successfully treated
-

Proprietary Technologies
Our proprietary technologies include OriCleverClover, which is a highly sensitive test with a low false positive rate. OriSelector precisely selects monitoring sites
| Testing Portfolio | Detection Technology | Specimen Type | Cancer Type |
|---|---|---|---|
| MiYuan OriMIRACLE LTM | BCR/TC rearrangement | Bone marrow fluid 、Peripheral blood、FFPE sample | Lymphatic hematologic tumor |
| OriMIRACLE STM | WES+MRD | Tumor tissue +ctDNA | All solid tumors |
partner
More Comprehensive Genomic Profile Testing
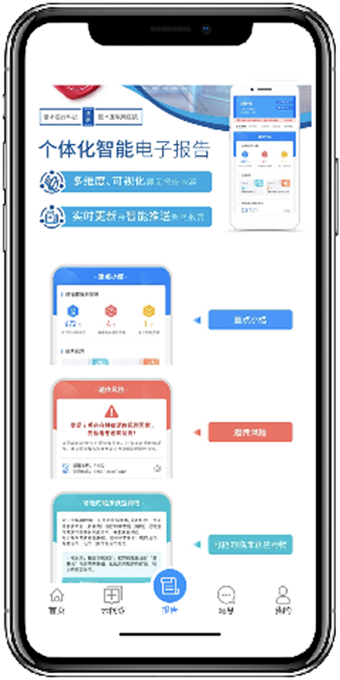
- We provide complete DNA and RNA sequencing services, generating high quality molecular and genomic data. This covers more than 50 clinical solid tumors and haematological tumors. This information helps clinicians make treatment decisions.
- We offer online ordering and digital reporting to smoothly communicate information to aid in clinical decision making.
-
YuanSu Series
700+gene solid tumor
Comprehensive Genomic Profiling Testing-
Yuansu®/YuansuLiquid™丨700+genesDNA sequencing for all solid tumor
-
YuansuS®丨DNA + RNA: 700 genesDNA+RNA sequencing for all solid tumor
-
YuansuIO™丨700+genesDNA+IHC sequencing for all solid tumor
-
-
More Next-Generation Sequencing Panels
40+— 20000+solid tumor
Comprehensive Genomic Profiling Testing-
Qing Yuan™ DNA + RNA | 400+genesDNA+RNA sequencing for hematopoietic and lymphoid tissue tumor
-
Zhi Xun WESPlus | 20,000+genesWhole Exome Test for all solid tumor
-
Ben Chu |3 genesDNA sequencing for breast/ ovarian cancer
-
-
Immunohistochemical platform
FDA-approved immunization checkpoint IHC testing
Internationally approved antibodies-
PD-L1 IHC TestingEvaluating PD-L1 protein expression from multiple tumor types
-
-

Contact address
 5th Floor, Building 3, 115 Xinjun Ring Road, Minhang District, Shanghai
5th Floor, Building 3, 115 Xinjun Ring Road, Minhang District, Shanghai Floor 4, Information Building 2, Zhaolou Road 3576 , Pujiang Town, Minhang District, Shanghai
Floor 4, Information Building 2, Zhaolou Road 3576 , Pujiang Town, Minhang District, Shanghai -

Customer Service Telephone
 400-821-9890
400-821-9890
Service Hours:9:00-18:00 Monday to Friday (except statutory holidays) -

Customer Service Email
 zbservice@origimed.com
zbservice@origimed.com
We will reply you as soon as we receive the email.

All rights reserved in © Copyright 2022 OrigiMed Ltd
Registration Number:沪ICP备16027109号-1


