OMSmartData Launch: China's Largest Pan-Cancer Real-World Database Focused on Pharmaceutical Enterprises
In the field of healthcare, the power of data is becoming increasingly prominent, serving as a key factor in driving innovation and accelerating drug development. As an innovative medical technology company driven by data, OrigiMed has launched OMSmartData, a new generation of precision oncology data analysis platform, leveraging its profound accumulation in the field of data.
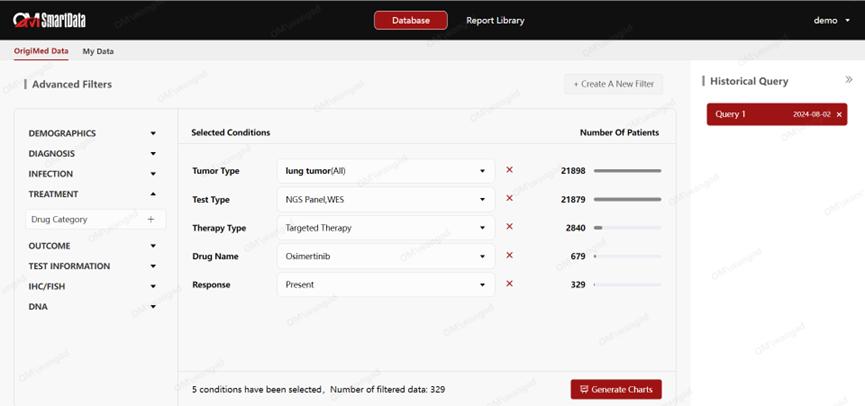
OMSmartData integrates real-world data to provide comprehensive data support to pharmaceutical companies, enabling them to make more accurate and efficient decisions in the process of oncology drug development and commercialization.
The Core Driving Force Behind New Drug Development
01
Comprehensive Data Coverage
02
Efficient Data Utilization
03
Compliance and Safety
Supporting the Entire Cycle of New Drug Development and Commercialization
· Preclinical/IND Stage: By analyzing the mutation rate of the target in the population and potential treatment plans after drug resistance, OMSmartData assists pharmaceutical companies in making more accurate decisions during the preclinical stage.
· Clinical Trial/Medical Stage: OMSmartData can rapidly identify patients who meet specific clinical trial criteria, accelerate the recruitment process, and support the analysis of combination therapies and indication selection.
· Commercialization Stage: Leveraging OMSmartData to estimate the potential of the target market, formulate medical, marketing, and commercial strategies, and help pharmaceutical companies achieve more effective market penetration after drug launch.
Case Studies in New Drug Development
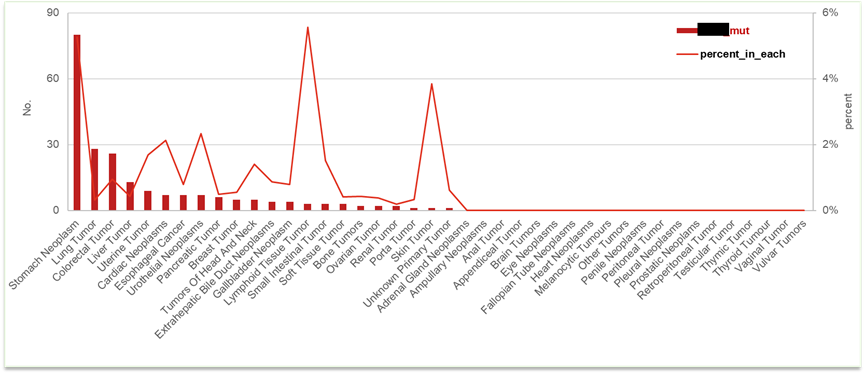
Case1: The market potential analysis for new drug X: Global biotechnology companies face challenges: how to assess the market potential of new drug X in China? OMSmartData provides in-depth market insights by analyzing the prevalence of specific genetic mutations and patient populations with related tumor types, helping companies evaluate the market potential of new drugs and formulate strategies.
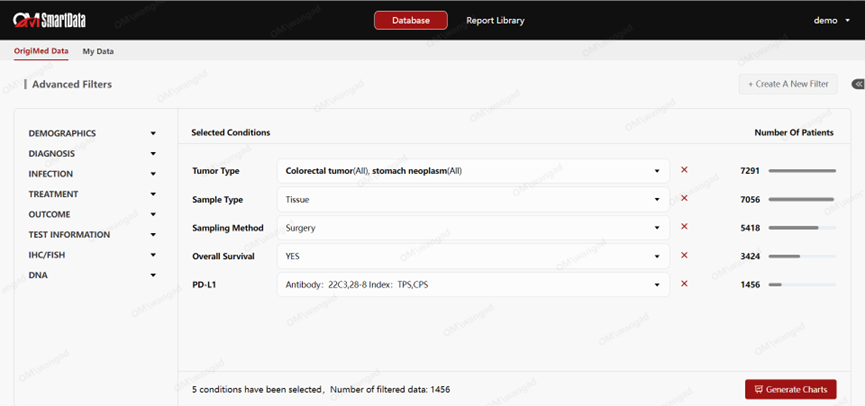
Case 2: New ADC Target X Data Support: One Chinese pharmaceutical company faces challenges in developing new ADC drugs: a lack of key data on Chinese patients. OMSmartData leverages its extensive data resources to provide data on the expression levels and prognostic effects of Target X in Chinese patients, supporting the IND application and subsequent development of the drug.
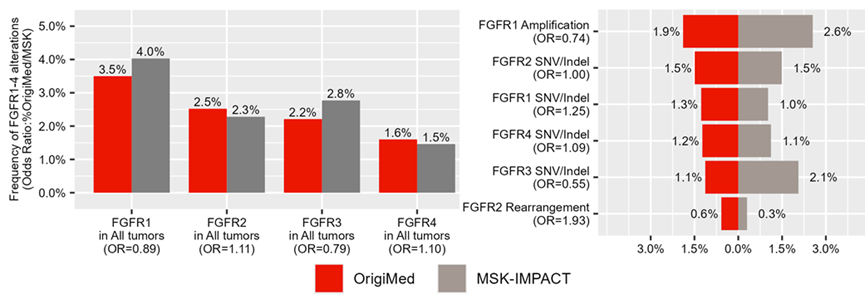
Case3: The Drug Prospects for China-Specific Cancers: Global pharmaceutical companies are keen to understand the potential of their drugs in the treatment of cancers that are prevalent in China. OMSmartData analyzes the distribution of specific targets/genes/biomarkers in cancer types such as nasopharyngeal carcinoma and liver cancer, which are characteristic of China, revealing the potential opportunities for drugs in these indications.
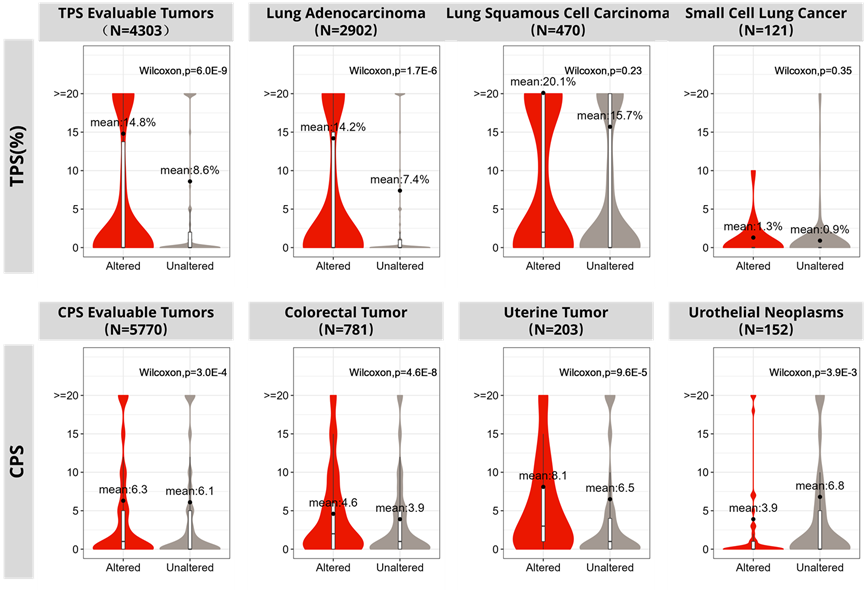
Case4: Possibilities for Drug Combination Therapies: Global pharmaceutical companies are exploring the potential of combining their targeted therapies with immune checkpoint inhibitors. OMSmartData provides data support by analyzing the correlation between specific biomarkers and drug response, assisting companies in designing more effective clinical trials.
Case 5: Study of Specific TKI Resistance Mechanisms: One Chinese pharmaceutical companies faces challenges when studying the resistance mechanisms to EGFR TKI therapy. OMSmartData analyzes genetic mutations in non-small cell lung cancer patients after receiving EGFR TKI treatment, revealing the mechanisms of resistance, and providing a scientific basis for subsequent treatment strategies.
OMSmartData
is not just a data analysis tool,
it
is a capable partner for pharmaceutical companies on the journey of oncology
drug development and commercialization.
We
look forward to joining hands with you,
推荐阅读
-
 点击查看详情 / Click for More Details.OrigiMed Showcases Latest Research Findings at the 2024 ESMO2024-09-11 11:30:00
点击查看详情 / Click for More Details.OrigiMed Showcases Latest Research Findings at the 2024 ESMO2024-09-11 11:30:00 -
 点击查看详情 / Click for More Details.2024 ASCO | Deepening Pharmaceutical Collaboration, Accelerating Drug Development with New Technologies and Multi-platform Advantages2024-06-05 09:34:00
点击查看详情 / Click for More Details.2024 ASCO | Deepening Pharmaceutical Collaboration, Accelerating Drug Development with New Technologies and Multi-platform Advantages2024-06-05 09:34:00 -
 点击查看详情 / Click for More Details.OrigiMed's SmartMTB Takes the Lead at the AACR 20242024-04-16 09:30:00
点击查看详情 / Click for More Details.OrigiMed's SmartMTB Takes the Lead at the AACR 20242024-04-16 09:30:00
All rights reserved in © Copyright 2022 OrigiMed Ltd
